Abstract
Introduction When pomalidomide was first approved by the European Medicines Agency in 2013 for the treatment of relapsed/refractory multiple myeloma (RRMM), a non-interventional post-authorization registry, the MM-015 POM PASS (NCT02164955), was set up to monitor the safety of patients with longer exposure to pomalidomide treatment in routine care. With the later approval of other treatments, including the anti-CD38 monoclonal antibody daratumumab, the MM treatment pathway has been changing (Darzalex summary of product characteristics. Janssen 2022). While the first indication of pomalidomide was for the treatment of RRMM in patients previously treated with ≥ 2 prior therapies, including lenalidomide and bortezomib, patients recruited in the MM-015 POM PASS study after daratumumab approval could potentially have been treated with daratumumab prior to receiving pomalidomide (Imnovid summary of product characteristics. Celgene 2020). This specific analysis therefore assessed safety and survival outcomes in the ongoing MM-015 POM PASS study of patients exposed to all 3 classes of drugs (TCE) and those not exposed to daratumumab (non-TCE).
Methods Patients receiving pomalidomide treatment were recruited into the registry from approximately 80 hematology/oncology sites in Europe. Patients should have received ≥ 1 dose of pomalidomide to be considered for the analysis. Patients who received ≥ 1 dose of lenalidomide, bortezomib, and daratumumab prior to receiving pomalidomide were classed as TCE; all other patients were classed as non-TCE. Adverse event (AE) incidence, duration of treatment (DoT), best overall response, and Kaplan-Meier estimates for overall survival (OS) were analyzed. A multivariate Cox regression was used to estimate adjusted hazard ratio (HR) for survival time between TCE and non-TCE subgroups.
Results The safety population had 761 patients; 85 were classed as TCE and 676 as non-TCE. At the data cutoff for this analysis (December 31, 2021), 2 TCE and 9 non-TCE patients remained on treatment. Patients in this study had a median age (range) of 71 (36-92) years, 53.6% were male, and 47.4% had an Eastern Cooperative Oncology Group performance status of 1. Baseline characteristics between TCE and non-TCE groups were broadly comparable; TCE patients had a higher median number of prior treatment lines (4.0 vs 3.0) and were more likely to have a high-risk cytogenetic abnormality (22.4% vs 9.8%) than non-TCE patients.
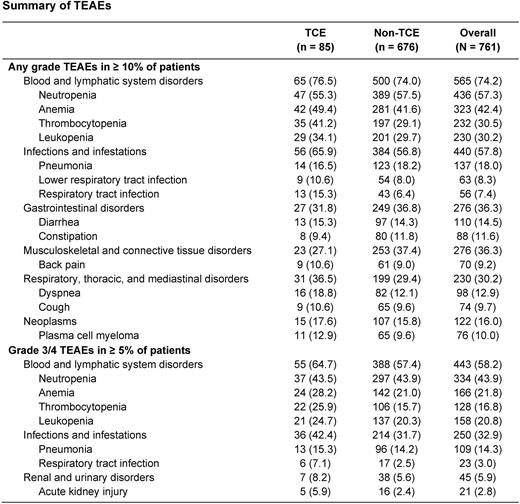
Most patients had ≥ 1 treatment-emergent AE (TEAE). TCE patients had higher rates of grade 3/4 TEAEs (95.3% vs 80.5%, respectively), serious TEAEs (75.3% vs 67.0%, respectively), and TEAEs leading to death (30.6% vs 22.9%, respectively) compared with non-TCE patients. Rates of drug-related TEAEs and TEAEs leading to dose reduction, interruption, or discontinuation were similar between TCE and non-TCE patients. Blood and lymphatic system disorders were the most common TEAEs for TCE and non-TCE patients at any grade (76.5% and 74.0%) or grade 3/4 (TCE, 64.7%; non-TCE, 57.4%) (Table). The main reasons for treatment discontinuation were progressive disease (TCE, 49.4% [n = 42]; non-TCE, 53.6% [n = 362]), AEs (TCE, 20.0% [n = 17]; non-TCE, 19.5% [n = 132]), and death (TCE, 16.5% [n = 14]; non-TCE, 10.4% [n = 70]). The death rate was higher in the TCE group, with 84.7% (n = 72) of the TCE group and 71.7% (n = 485) of the non-TCE group having died.
TCE and non-TCE patients had a similar median DoT [range] (5.2 [0.3-43.0] vs 5.1 [0.2-74.6] months) and overall response rate (35.3% [n = 30] vs 36.7% [n = 248]). OS was lower in TCE patients compared with non-TCE patients (9.8 vs 15.6 months; HR, 1.73; 95% confidence interval [CI], 1.33-2.23; P < 0.0001).
Conclusions Updated results of this ongoing non-interventional study continue to show that the safety profile of pomalidomide-based treatment in patients with RRMM in the real world is consistent with that from randomized controlled trials. No new safety signals were identified for patients treated with pomalidomide even when being exposed to bortezomib, lenalidomide, and daratumumab. TCE patients in the study had a higher risk of death compared with those who were non-TCE (HR, 1.73; 95% CI, 1.33-2.23).
Study support The study was supported by Celgene, a Bristol-Myers Squibb Company.
Disclosures
Ramasamy:Takeda, Bristol Myers Squibb, Janssen, Amgen, GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding, Speakers Bureau; Sanofi, Adaptive biotech, Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Abildgaard:Bristol Myers Squibb, Janssen, Takeda, Amgen: Research Funding. Gamberi:Amgen, Bristol Myers Squibb, Janssen, GlaxoSmithKline: Honoraria; Takeda, Bristol Myers Squibb, GlaxoSmithKline, Sanofi, Janssen, Amgen: Other: Advisory board. Millar:Bristol Myers Squibb: Current Employment. Ansaloni:Bristol Myers Squibb: Current Employment. Atiba-Davies:Bristol Myers Squibb: Current Employment. Bernasconi:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Plesner:Janssen, Celgene, Takeda, Oncopeptides, Genentech, CSL Behring, AbbVie: Membership on an entity's Board of Directors or advisory committees; Janssen, Genmab, Celgene, Takeda, Oncopeptides, Genentech, AbbVie, Roche, Bristol Myers Squibb: Research Funding. Dhanasiri:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal